Table of Contents
Childhood Absence Epilepsy
- Childhood absence epilepsy (CAE) is an age-dependent, idiopathic form of generalised epilepsy (IGE) characterised by multiple absence seizures per day, as well as bilateral, symmetrical, and synchronous discharges of 3-Hz generalised spike and waves (GSW) in the electroencephalogram.
- The 2017 International League Against Epilepsy (ILAE)classification suggested that the term IGE could be reserved for the four syndromes including childhood absence epilepsy (CAE), juvenile absence epilepsy (JAE), Juvenile Myoclonic Epilepsy (JME), and epilepsy with generalized tonic–clonic seizures alone (GTCA)[1].
- CAE makes for 2% to 10% of all paediatric epilepsies and 8-15% of school-aged childhood epilepsies. Seizures occur many times daily and consist of brief staring spells, sometimes with rhythmic eye blinking or motor automatisms, lasting seconds, with immediate return to the baseline level of awareness and activity. Children with CAE develop normally, although attentional deficiencies or other subtle behavioural or cognitive abnormalities may be present at onset.
Investigations
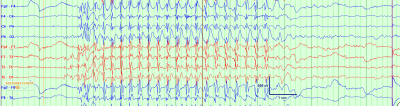 Fig. 1: A typical absence seizure on electroencephalogram, characterized by 3 Hz generalized spike wave discharges, with abrupt onset and offset, lasting several second
Fig. 1: A typical absence seizure on electroencephalogram, characterized by 3 Hz generalized spike wave discharges, with abrupt onset and offset, lasting several second
- On electroencephalography (EEG), seizures are characterized by a highly recognizable pattern of generalized (bilateral, symmetric and synchronous) 3 Hz spike and wave discharges. See figure 1
- Consider the possibility of Glucose transporter 1 deficiency syndrome in a child who has absence seizures that started before the age of 4 years or who has absence seizures along with an abnormal neurologic exam or significant developmental delays.
Treatment
Three antiepileptic medications, namely ethosuximide (ETX), valproic acid (VPA), and Lamotrigine (LTG), have traditionally been the primary choices for treating childhood absence epilepsy (CAE). The 2010 Childhood Absence Epilepsy research presented conclusive evidence, classified as class I, supporting the use of ETX as the most effective initial treatment for CAE[2]. See algorithm figure 2.
| Summary of medications used for childhood absence epilepsy | |||
|---|---|---|---|
| Name | Initial dose | Maintenance dose | Maximum dose |
| Ethosuximide | 10–15 mg/kg/day | 20–30 mg/kg/day | 40 mg/kg/day up to 2 g/day |
| Valproate | 10–15 mg/kg/day | 20–30 mg/kg/day | 60 mg/kg/day up to 3 g/day |
| Lamotrigine | For patients not taking valproate or other enzyme inducers: 0.3 mg/kg/day | For patients not taking valproate or other enzyme inducers: 4.5–7.5 mg/kg/day | For patients not taking valproate or other enzyme inducers: 300 mg/day |
| For patients taking valproate: 0.15 mg/kg/day | For patients taking valproate: 1–5 mg/kg/day | For patients taking valproate: 200 mg/day | |
| For patients taking enzyme inducers and NOT valproate: 0.6 mg/kg/day | For patients taking enzyme inducers and NOT valproate: 5–15 mg/kg/day | For patients taking enzyme inducers and NOT valproate: 400 mg/day | |
| Clobazam | <30 kg: 5 mg/day | <30 kg: 10–20 mg/day | <30 kg: 40 mg/day |
| >30 kg: 10 mg/day | >30 kg: 40 mg/day | >30 kg: 60–80 mg/day | |
| Levetiracetam | 20–30 mg/kg/day | 40 mg/kg/day | 60–90 mg/kg/day up to 3 g/day |
| Topiramate | 1–3 mg/kg/day | 5–9 mg/kg/day | 15 mg/kg/day up to 1600 mg/day |
| Zonisamide | 1–2 mg/kg/day | 5–8 mg/kg/day | |
Fig. 2: Treatment Algorithm for Childhood Absence Epilepsy
- Because VPA slows down the metabolism of LTG by blocking liver enzymes, titration of LTG starts at an even lower dose, moves more slowly, and hits a lower target in a person who is also taking VPA than in a person who is not taking any other medication.
- There are important cognitive, behavioural, and psychological problems associated with CAE that need to be identified early and dealt with. Anxiety and attention deficit hyperactivity disorder (ADHD) are significantly associated with CAE[3].
Differentiating between CAE and JAE
- Age at onset of CAE is considered to be 2 to 13 years of age and for Juvenile Absence Epilepsy (JAE) to be 8 to 20 years of age; there is five years of overlap[4].
- Patients with JAE more often have GTCS and more frequently experience seizure-related injuries compared with patients with CAE
- Valproate can be considered as the drug of choice in men and lamotrigine as the first drug of choice in women with JAE. See table 1
| Feature | CAE | JAE |
|---|---|---|
| Age at onset | ||
| Usual | 4–10 years | 9-13 yrs |
| Range | 2–13; caution if diagnosing at <4yrs of age | 8–20 years; exceptional cases may present in adulthood |
| Development | Typically normal, but may have learning difficulties or ADHD | Typically normal, but may have learning difficulties or ADHD |
| Absences | ||
| Frequency | At least daily to multiple per day but may be underrecognized by family | less than daily |
| Duration | Typical duration = 3–20 s | Typical duration = 5–30 s |
| Impaired awareness | Severe loss of awareness | Less complete impairment of awareness |
| Other seizure types | ||
| Febrile | Occasional | Occasional |
| Generalized tonic-clonic seizures | Rarely precede or occur during period of frequent absences but may occur later with evolution to other IGE syndrome | May precede and commonly occur during the period of frequent absences |
| Myoclonic | Prominent myoclonus exclusionary | Prominent myoclonus exclusionary |
| EEG background | OIRDA in 21% | Normal |
| Interictal epileptiform discharge | ||
| Awake | 2.5–4-Hz generalized spike-wave | 3–5.5-Hz generalized spike-wave |
| Asleep | Polyspike and wave may be seen in drowsiness and sleep only | |
| Irregular generalized spike-wave | Uncommon | More common than CAE |
| Photoparoxysmal response | Rare IPS triggers generalized spike-wave in 15%–21% but does not induce seizures | Rare IPS triggers generalized spike-wave in 25% but does not induce seizures |
| Hyperventilation induction | 87% | 87% |
| ICTAL EEG | Regular 3-Hz (range = 2.5–4 Hz) generalized spikewave; 21% may have absences starting at 2.5-Hz spike-wave, and 43% may have absences starting at 4 Hz; if no generalized spike-wave is seen with hyperventilation for 3 min in an untreated patient, CAE can be excluded Disorganized dischargesa less frequent | Regular 3–5.5-Hz generalized spike-wave If no generalized spike-wave is seen with hyperventilation for 3 min in an untreated patient, JAE can be excluded Disorganized dischargesa 8 times more frequent than CAE |
| Abbreviations: ADHD, attention-deficit/hyperactivity disorder; CAE, childhood absence epilepsy; EEG, electroencephalogram; IGE, idiopathic generalized epilepsy; IPS, intermittent photic stimulation; JAE, juvenile absence epilepsy; OIRDA, occipital intermittent rhythmic delta activity. a Disorganized discharges are defined as either brief (less than 1 sec and and transient interruptions in ictal rhythm or waveforms of different frequency or morphology during the ictal rhythm. | ||
While reporting an EEG the following terms are suggested, whenever appropriate: spike-and-slow-wave complex, 3 c/s spike-and-slow-wave complex, sharp-and-slow wave complex. Use of the term “absences” is discouraged when describing EEG patterns.
Pathophysiology
- Absence seizures result from disruptions in thalamocortical rhythms via T-type calcium channel dysfunction.
see also Absence Seizures
Genetics
- There are few genes that confer monogenic risk for CAE, found through family studies or large cohort studies (e.g., GABRG2, GABRA1, SLC2A1[7]).
- Several recurring Copy Number Variations (CNVs), such as 15q11.2, 15q13.3, and 16p13.11 microdeletion[9], contribute to a complicated inheritance pattern seen in CAE.A chromosomal microarray should be requested for children with substantial learning difficulties due to the higher probability of pathogenic CNVs
Treatment resistant CAE
- If first- and second-line drugs don't work, clobazam might be worth a try. However, there isn't much literature on its use in treatment-resistant CAE
- The ketogenic diet has also been used successfully in children with treatment-resistant CAE[10]
References
[PMID: 28276062] [PMCID: 5386840] [DOI: 10.1111/epi.13709]
[PMID: 30734897] [PMCID: 6394437] [DOI: 10.1007/s40272-019-00325-x]
[PMID: 18557780] [DOI: 10.1111/j.1528-1167.2008.01680.x]
[PMID: 35503716] [DOI: 10.1111/epi.17236]
[PMID: 30856420] [PMCID: 6573015] [DOI: 10.1016/j.eplepsyres.2019.02.013]
[PMID: 24684814] [DOI: 10.1016/j.seizure.2014.03.002]
[PMID: 19798636] [DOI: 10.1002/ana.21724]
[PMID: 28165634] [PMCID: 6171340] [DOI: 10.1002/ana.24886]
[PMID: 19843651] [PMCID: 2801323] [DOI: 10.1093/brain/awp262]
[PMID: 20647578] [DOI: 10.1177/0883073810376443]
[PMID: 21320119] [DOI: 10.1111/j.1528-1167.2010.02976.x]
[PMID: 18808424] [DOI: 10.1111/j.1469-8749.2008.03099.x]
[PMID: 21680209] [DOI: 10.1016/j.ejpn.2011.05.007]